Rare Diseases Clinical Research Network (RDCRN)
The Rare Diseases Clinical Research Network (RDCRN) program is designed to advance medical research on rare diseases by providing support for clinical studies and facilitating collaboration, study enrollment and data sharing.
About the RDCRN
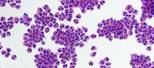
Human eosinophils, a type of white blood cell, are shown isolated from blood. In patients with eosinophilic esophagitis, these cells store and release packages of inflammatory proteins (red) that can damage the throat and esophagus. (Cincinnati Children’s Hospital Medical Center Photo/Julie Caldwell)
The Rare Diseases Clinical Research Network (RDCRN) program advances medical research on rare diseases by providing support for clinical studies and facilitating collaboration, study enrollment and data management. Through a network of consortia, scientists from multiple disciplines at hundreds of clinical sites around the world work in partnership with patients and patient advocacy groups to study more than 280 rare diseases. The program is federally mandated by Congress: The Rare Diseases Act of 2002 directed the Division of Rare Diseases Research Innovation to support regional "RDCRCs of Excellence" for clinical research, career enhancement, and demonstration of diagnostic, prevention, control and treatment methods for rare diseases.
The RDCRN is designed to promote highly collaborative, multi-site, patient-centric, translational and clinical research. The Rare Diseases Clinical Research Consortia (RDCRCs) focus on unmet clinical trial readiness needs that will move the field of research forward from its current state to one closer to successfully developing treatments for patients.
The RDCRN facilitates clinical research in rare diseases through support for consortia via:
- Collaborative activities, including multisite longitudinal studies of individuals with rare diseases and/or clinical trials
- Career enhancement — encouraging the next generation of rare disease researchers
- Pilot and feasibility projects
The consortia within the RDCRN are supported by a Data Management and Coordinating Center (DMCC) that provides harmonized network wide:
- Data management to facilitate high quality data standards, collection, storage and sharing
- Clinical research support
- Access to information about rare diseases for basic and clinical researchers, academic and practicing physicians, patients and the public
RDCRN Clinical Research
RDCRN consortia support a broad range of clinical research, including clinical trial readiness, natural history studies, identification of biomarkers and outcome measures, and early phase clinical trials.
A Collaborative Environment
Funding and scientific oversight for the RDCRN are provided by NCATS and ten other NIH entities: the Eunice Kennedy Shriver National Institute of Child Health and Human Development; the National Heart, Lung and Blood Institute; the National Institute of Allergy and Infectious Diseases; the National Institute of Arthritis and Musculoskeletal and Skin Diseases; the National Institute of Dental and Craniofacial Research; the National Human Genome Research Institute; the National Institute on Aging; the National Institute of Neurological Disorders and Stroke; the National Institute on Deafness and Other Communication Disorders and the Office of Research on Women’s Health. In addition, patient advocacy organizations may contribute funding.
Data sharing is a key aspect of the RDCRN framework and a priority for many stakeholders involved with the network and the RDCRN Data Management and Coordinating Center provides non-binding guidance to consortia on general principles for data sharing and key factors to consider when developing data sharing policies. For more information, review the RDCRN Data Sharing Guidance (PDF - 235KB).
RDCRN News

Rare Diseases Clinical Research Network Awardees Expand the Reach of NIH Impact
February 5, 2026 - NCATS News
- Rare Diseases Clinical Research Network (RDCRN)
NIH awarded funding to 10 new and five continuing Rare Diseases Clinical Research Network (RDCRN) consortia to help address challenges in rare diseases treatment development.
Read ArticleUNC Researchers Awarded $7.3 Million to Advance Research on Rare Bronchiectatic Diseases
February 4, 2026 - Grantee/Partner News
- Rare Diseases Clinical Research Network (RDCRN)
A Rare Respiratory Disease May Be More Prevalent in Quebec
November 27, 2025 - Grantee/Partner News
- Rare Diseases Clinical Research Network (RDCRN)
The Shift to Precision Medicine in Myasthenia Gravis
November 17, 2025 - Grantee/Partner News
- Rare Diseases Clinical Research Network (RDCRN)
I Am Translational Science Video
Child neurologist Mustafa Sahin, M.D., Ph.D., shares how advances in translation helped his team provide new treatments to young patients with rare neurodevelopmental disorders.
RDCRN Open Funding Opportunities

Find open funding opportunities for RDCRN.
Related Research

Rare Diseases Registry Program (RaDaR)
We provide easily accessible guidance to the rare diseases community on how to set up and maintain high-quality registries.

Bespoke Gene Therapy Consortium (BGTC)
The Bespoke Gene Therapy Consortium focuses on developing platforms and standards to speed the development and delivery of gene therapies for rare diseases.

Toolkit
Our Toolkit for Patient-Focused Therapy Development is a collection of online resources to assist patient groups in the research and development of new treatments.