Spotlight on Collaboration: A Journey From Biological Probes to Potential Therapeutics
Researchers studying the biology of diseases often use scientific tools called biological probes, which are small molecules that change biological processes or characteristics of a disease in some way. By using probes to increase or decrease the activity of a particular protein in a test called an assay, scientists can explore that protein’s role in health and disease. Sometimes, probes that produce a desired activity in an assay become a starting point for new therapeutics to treat disease. Traditionally, pharmaceutical and biotechnology companies have had the knowledge and extensive resources needed to develop successful probes.
That story changed when what is now the NCATS Chemical Genomics Center (NCGC) was established in 2004. NCGC was the first center in a network of small molecule screening and medicinal chemistry optimization sites that produced small molecule probes as part of the NIH Common Fund’s Molecular Libraries Program.
Over the past decade, NCGC’s scientists have worked closely with academic, nonprofit and biotech researchers on more than 300 collaborative probe development projects in virtually every area of biology and disease. Together, the partners have developed biological assays that are screened against hundreds of thousands of compounds using NCATS’ state-of-the-art, high-throughput (large-scale) screening robots. The identified compounds are then turned into probes via medicinal chemistry optimization.
“We want to help investigators with a promising idea about important new biology or a novel way to reverse a disease state to develop the biological probes needed to test that idea,” said NCATS Director Christopher P. Austin, M.D. “Catalyzing the development of such innovative translational tools and technologies that speed development of new treatments for patients is NCATS’ mission.”
As a demonstration of this mission in action, an NCATS-catalyzed project team has explored an enzyme family called lipoxygenases (LOX), which help the body metabolize fatty acids. What began as a project to explore the fundamental science has developed into a series of disease-focused collaborations among NCATS scientists and several U.S. research institutions. The team’s efforts culminated in the discovery of three novel small molecule chemical probes with the potential to treat diabetes, stroke and thrombosis.
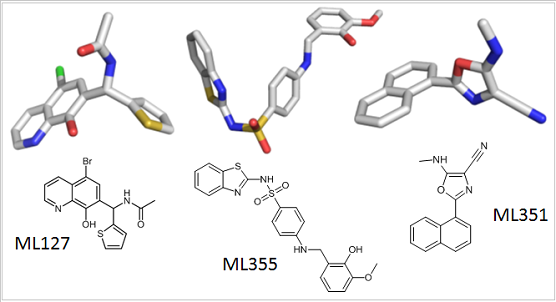
The chemical structures of small molecule probes used by scientists to understand the role of the lipoxygenase family of enzymes in health and disease.
A Collaboration Is Born
The collaboration began in 2006 when Ted Holman, Ph.D., an enzymologist at the University of California, Santa Cruz, teamed with NCGC researchers David Maloney, Ph.D., Anton Simeonov, Ph.D., and Ajit Jadhav to develop molecular probes that would inhibit (block) the action of various LOX enzymes. Too much LOX activity may underlie a wide range of diseases, including asthma, heart disease, stroke and diabetes. By decreasing LOX activity in cell cultures and animal disease models, inhibitors could provide powerful tools for understanding the roles those enzymes play in health and disease.
Until he joined forces with the NCGC team, Holman had trouble finding high-quality LOX inhibitors to use as molecular probes. Existing inhibitors were not specific enough, meaning that they acted on other enzymes in addition to the LOX target enzyme.
Around that same time, a colleague told Holman about NCGC, where experts conducted screens using a method that involves testing compounds at multiple concentrations instead of just one. Screening at multiple concentrations allows for a higher probability of finding potential “hits,” which is particularly useful for difficult targets such as the LOX enzymes. Holman applied to work with the NCGC team through the Molecular Libraries Program.
Through close collaboration over the past seven years, a joint project team made up of the Holman laboratory and NCGC team members successfully discovered and optimized several inhibitors for the LOX enzymes. Shortly after the team published initial results, biologists studying the role of LOX in disease were lining up, seeking collaborations with Holman and the NCGC team to use the LOX inhibitors for their studies. Up to that point, these scientists had never been able to find good LOX probes to adequately explore their diseases of interest. “What they needed was a small molecule tool to corroborate their molecular genetic data, to show that the LOX enzyme was involved in the diseases they were studying,” Holman said. “That’s the gold standard.”
A New Tool for Solving Research Problems
One of these biologists was Jerry Nadler, M.D., at Eastern Virginia Medical School in Norfolk. From his work with a member of the LOX family called 12-LOX, using human tissue and mice, he had found that the enzyme’s activity was involved in the development of diabetes. Eliminating 12-LOX from mice ultimately protected them from diabetes.
“The research had been going very well, but we were at a roadblock,” Nadler said. “To move the research forward, and possibly into the clinic, we needed a way to inhibit this enzyme. There was nothing available that was specific or good enough.”
Nadler soon began working with two high-quality 12-LOX inhibitors (called ML355 and ML127) developed by Holman and the NCGC team. Nadler found that ML355 and ML127 protected pancreatic insulin-producing cultured cells from diabetic damage. The work led to funding from the Juvenile Diabetes Research Foundation, which will enable Nadler to treat diabetic mice with the inhibitor and assess its therapeutic potential. “It will be the first step on the way to clinical trials,” Nadler said.
Broadening the Role of LOX Inhibitors
Michael Holinstat, Ph.D., a biologist at Thomas Jefferson University in Philadelphia, also was examining the 12-LOX enzyme, but in a different condition: thrombosis, the formation of clots that can lead to heart attack and stroke. He had found evidence that 12-LOX was involved in the activation of platelets, the blood cells that form clots.
Through his collaboration with Holman and the NCGC team, Holinstat used ML355 and other related compounds to show that 12-LOX is involved in platelet activation. Within five years, this work led to grants from the American Heart Association, National Institute of General Medical Sciences, and National Heart, Lung, and Blood Institute. This funding enables Holinstat to further explore the therapeutic potential of 12-LOX inhibitors to prevent thrombosis in at-risk patients.
According to Holinstat, this success is due in no small part to the team-based approach at NCGC. “In this day and age, we can’t work in a lab by ourselves” he said. “I’m not a medicinal chemist. I don’t do high-throughput screening, so I would not have gotten to the point of identifying inhibitors for this enzyme.
Likewise, NCATS and the Holman group are not disease experts. It’s a symbiotic relationship. [The collaboration] has allowed our lab to go in directions we had not otherwise been able to even think about.”
An Expanding Collaboration
Klaus van Leyen, Ph.D., a stroke researcher at Harvard, had found evidence that another member of the LOX family, 15-LOX-1, contributed to brain cell death and damage after stroke, but he needed an inhibitor to test his hypothesis. van Leyen began working with Holman and the NCGC team to develop an inhibitor for 15-LOX-1. They found one called ML351, and van Leyen began using it to study stroke.
van Leyen found that ML351 reduces brain injury in mouse models of stroke. He continues to work with the team to optimize ML351’s effectiveness, so that one day soon it could be tested in humans. van Leyen has also received funding to study the therapeutic potential of LOX inhibitors in cardiac arrest.
“To go from a random library of compounds you know almost nothing about and take it all the way to animal models of stroke has been fascinating,” van Leyen said. “This kind of collaboration has a synergistic effect, allowing us to move forward much more quickly than we could with any other type of approach.”
From One Probe to Multiple Disease Applications
The LOX teamwork exemplifies the kind of collaborative, innovative approaches to making translational research more efficient that are at the heart of the NCATS mission.
“This collaboration has restructured my lab in incredible, positive ways,” Holman said. “That’s the power of NCATS: I run a small lab in a middle-sized university. NCATS resources have enabled me to play in a big field and stand taller than my stature would normally allow. NCATS was the catalyst for making it all happen.”
Posted June 2014