NCATS Support Leads to Clinical Trial to Test Repurposed Cancer Treatment as Alzheimer’s Therapy
As Baby Boomers get older, the number of people with age-related conditions such as cancer and Alzheimer's disease continues to grow. Alzheimer's disease is the most common form of dementia, a group of disorders that cause progressive loss of memory and other mental processes. About 5 million Americans have Alzheimer's disease, and current drug therapies can only ease symptoms of the disease without stopping its progression. New treatments — so-called disease-modifying therapies — are needed to halt Alzheimer's by targeting its underlying mechanisms.
Blocking that path to therapeutic success is the costly, complex process of drug development. The average length of time from discovery of a therapeutic target to approval of a new drug is about 14 years. The failure rate during this process exceeds 95 percent.
NCATS is addressing these translational bottlenecks through programs such as the Discovering New Therapeutic Uses for Existing Molecules (New Therapeutic Uses) program. Launched in 2012, this initiative matches academic researchers with pharmaceutical industry assets that have undergone significant research and development to accelerate the process of finding new therapies.
Now, NCATS is celebrating one of the first promising results from the New Therapeutic Uses program: Center-supported scientists at Yale University School of Medicine have found that an experimental compound originally developed as a cancer therapy potentially could be used to treat Alzheimer's disease. The compound successfully reversed brain problems in mouse models of the condition, and now the researchers are testing it in humans. The results of the animal study were published for early view on March 21, 2015, in the Annals of Neurology. Read the NIH news release.
A New Therapeutic Target
Back in 2012, Yale neurobiology researcher, neurologist and senior author of the animal study Stephen Strittmatter, M.D., Ph.D., and his colleagues found an important, missing piece of the Alzheimer's puzzle. They wanted to understand more completely the molecular events that occur in the brain to produce symptoms.
In Alzheimer's disease, abnormal clumps of amyloid beta protein build up in the brain. These protein clusters damage brain cells (neurons), eventually killing them. However, how amyloid beta harms cells has been unclear.
The Yale team discovered that aggregated amyloid beta activates a series of signals within neurons that leads to abnormal functioning and loss of synapses, which are the spaces between neurons that enable the cells to "talk" to each other and form memories. Central to this process is the activation of a protein called Fyn kinase through another molecule, cellular prion protein. These results suggested that a compound that blocks Fyn activity might represent a potential disease-modifying therapy for Alzheimer's.
The Power of Crowdsourcing
Around the time Strittmatter's Fyn finding emerged, NCATS launched the New Therapeutic Uses program and released a list of pharmaceutical industry assets, inviting scientists to pitch new ideas for diseases that might be treated with those assets. That 2012 list included a Fyn kinase inhibitor, saracatinib (AZD0530 [PDF - 65KB]), developed by biopharmaceutical company AstraZeneca. Strittmatter, along with co-principal investigators Haakon Nygaard, M.D., Ph.D., and Christopher van Dyck, M.D., submitted a proposal to test the hypothesis that saracatinib could improve Alzheimer's-related brain abnormalities. The team received one of the first New Therapeutic Uses awards in June 2013.
"AstraZeneca developed saracatinib to treat cancer outside the brain, so nobody had thought to link it to Alzheimer's disease," Strittmatter said. "This connection — between our knowledge of Fyn kinase in the brain and AstraZeneca's information on this compound — would never have happened without the New Therapeutic Uses program."
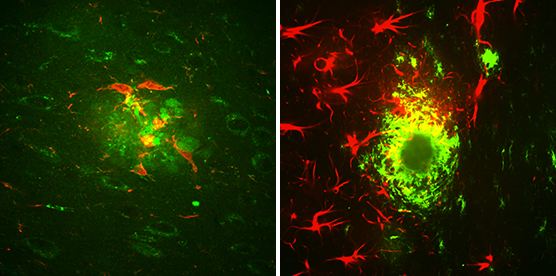
Two types of brain cells, microglia (red, left) and astrocytes (red, right), surround amyloid beta clusters (green) located in the brain of a mouse that exhibits Alzheimer’s-like symptoms. (Credit: Strittmatter Laboratory, Yale University Photo/Adam Kaufman)
A Promising Effect in Mice
AstraZeneca scientists teamed with the Yale group, providing saracatinib for the mouse study and sharing knowledge and data gathered from previous studies.
"No one individual or group has complete knowledge of disease pathways and treatment targets," said Craig Wegner, Ph.D., Head, Boston Emerging Innovations Unit, Scientific Partnering & Alliances within AstraZeneca's Innovative Medicines and Early Development Biotech Unit. "This program successfully unites scientists from government, academia and industry and is a great example of how we are working together to push the boundaries of science."
The Yale team gave the experimental drug to mice with Alzheimer's-like symptoms, such as memory problems and age-related buildup of abnormal amyloid beta clusters, modeling the development of the disease in humans. After four weeks, the Alzheimer's mice showed complete reversal of spatial learning and memory loss. When the scientists examined the brains of the mice, they found that the characteristic synapse loss had been fully restored, providing a biological explanation for the memory improvement.
The treatment also reduced several other Alzheimer's-related biochemical changes in the mice and did not appear to be toxic. Although many experimental treatments have aimed to reduce abnormal amyloid beta buildup in the brain, this one is unique in that it targets the toxic effects of the protein clusters within a cell, appearing to protect it from damage.
The Yale research team also has completed a successful Phase 1b safety study of saracatinib in humans with Alzheimer's disease, showing that the compound reaches the brains of patients at levels similar to those beneficial in mice. The study results have been accepted for publication later in 2015. The data are encouraging, but the researchers cautioned that more human studies are needed to determine if saracatinib is an effective treatment for Alzheimer's.
An Accelerated Path to the Clinic
Saracatinib's prior development and the Yale team's successful completion of animal and human studies enabled the compound to advance rapidly into a larger, multisite Phase 2a trial in Alzheimer's patients now ongoing. "The speed and efficiency with which this research has advanced has set new standards of excellence, enabling us to jointly push the boundaries of medical science," Wegner said.
Using the pre-negotiated NCATS template agreements, which were designed to streamline the legal and administrative process for research collaboration by multiple organizations, "really facilitated our collaboration with Yale, so scientists could be scientists," he added.
"Through this project, NCATS and AstraZeneca have provided us with an incredible shortcut in the drug development process and have accelerated the path to finding a more effective Alzheimer's disease treatment," Strittmatter said.
In the Phase 2a trial, 152 participants will receive saracatinib or placebo for one year. Researchers will assess safety, tolerability and effectiveness of the experimental drug, and they will use brain imaging to visualize the effect of saracatinib on synapse function — the same feature improved by the drug in mice. Study investigators currently are enrolling older adults with Alzheimer's disease to participate in the trial and expect to have results in about two years. Learn more about the trial via ClinicalTrials.gov.
Both human trials were funded by the New Therapeutic Uses program. The Phase 2a study will take place at multiple clinical sites as part of the Alzheimer's Disease Cooperative Study, an initiative for multisite studies sponsored by the National Institute on Aging (NIA) to facilitate the development and testing of new therapeutics for the condition. In addition to funding from NCATS, the NIH Common Fund, NIA, BrightFocus Foundation, Alzheimer's Association and Falk Medical Research Trust provided support for the animal study.
"The Yale team's awareness of this new Alzheimer's drug target, combined with AstraZeneca's drug development resources, allowed the rapid advancement of saracatinib to clinical testing, demonstrating the power of NCATS' New Therapeutics Uses crowdsourcing approach," said NCATS Director Christopher P. Austin, M.D. "By reengineering the drug development pipeline through projects like this, we can more quickly deliver new and better treatments to patients."
Posted March 2015