NCATS and Johns Hopkins Researchers Identify New Therapeutic Strategy for Eye Diseases
Understanding of the causes and characteristics of many diseases has improved dramatically over the past several decades, but in most cases, new interventions based on this understanding have not kept pace. NCATS focuses on ways to speed the development of diagnostics and therapeutics to unlock the promise of science for patients. The NCATS approach — which is based on innovation in how science is done as well as what is done — now has led to dramatic success for a project focused on degenerative diseases of the retina. Retinal diseases, including glaucoma, retinitis pigmentosa and age-related macular degeneration (AMD), damage the vision of millions of people worldwide. The work was published in the March 5, 2013, issue of the Proceedings of the National Academy of Sciences (PNAS).
Like many other diseases, glaucoma typically has limited or no treatment options. Although researchers possess a breadth of knowledge about the cause of glaucoma, they have struggled to identify molecular targets that could lead to new and more effective treatments. Scientists at NCATS are experts at this first step in the translational process, determining targets for disease intervention. However, to be effective in addressing specific diseases, NCATS’ translational expertise is matched with expertise in those diseases — thus, the fundamental NCATS principle that "translation is a team sport." NCATS custom-assembles a team for every project, each built to solve a particular roadblock to translation.
In this case, the team included NCATS and the Johns Hopkins School of Medicine in Baltimore. Don Zack, M.D., Ph.D., a glaucoma specialist and molecular biologist at Hopkins’ Wilmer Eye Institute, had extensive knowledge and robust animal models of retinal degenerative diseases. He and his team, which included Derek Welsbie, M.D., Ph.D., Zhiyong Yang, M.D., Ph.D., Cindy Berlinicke, Ph.D., and John Fuller, Ph.D., identified several compounds that appeared to stop the death of retinal ganglion cells (RGCs), the neurons in the back of the eye that, when damaged in glaucoma, lead to vision loss and blindness.
Jim Inglese, Ph.D., an expert in cell signaling and assay technologies who directs NCATS’ Assay Development and Screening Technology Laboratory, led the NCATS part of the glaucoma team.
Together, the investigators adapted an assay, or test, developed at Hopkins to screen compounds on a much larger scale, including specialized "kinase inhibitor" compounds from GlaxoSmithKline that Inglese had studied extensively. Using these large compound collections and the improved assay, the Hopkins-NCATS team identified many new compounds that showed promising activity in preventing RGC death.
But with this success came the next translational roadblock: the need to determine how the compounds worked to prevent cell death.
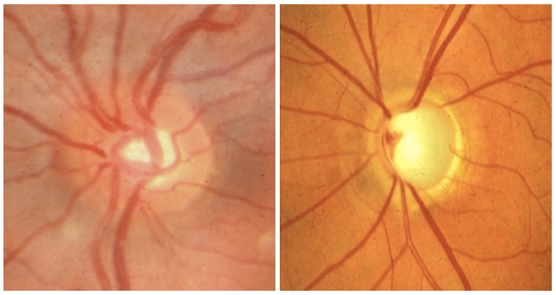
The image on the left shows the optic disc from a normal eye. The image on the right shows the disc from an eye in which the optic nerve has been damaged by glaucoma.
Overcoming this roadblock required specialized expertise, so the team followed the NCATS playbook and asked another scientist to join them: Scott Martin, Ph.D., who leads NCATS’ RNA interference (RNAi) initiative. Scientists use RNAi to investigate the function of genes, reveal networks of genes involved in health and disease, and identify molecular targets for intervention. Using an RNAi technique involving molecules called small interfering RNAs (siRNAs), Martin and his colleagues can turn down every gene in the human genome one by one, gaining unprecedented insights into the full collection of genes involved in a disease. In this case, Martin and Welsbie used RNAi to identify the target of the compounds Inglese and Zack had identified. To do so, the team had to employ yet another new technology: RNAi screening in “primary” RGC cells. These cells are taken directly from a retina rather than artificial cell lines cells created in a lab.
"Researchers typically use standard cell lines in RNAi studies, but they aren’t the most accurate models of disease," explained Welsbie, first author on the PNAS paper. Researchers have used primary cultures of mouse RGCs for a wide variety of studies, but scientists had never used primary RGCs successfully in an RNAi screen. Working together, the Hopkins-NCATS team successfully delivered the siRNAs to RGCs by attaching them to magnetic nanoparticles and using a magnet to draw the nanoparticles into the cells. The team must conduct further studies using this new method to determine if it will work on other primary cell types. If successful, this advance could help meet the translational need for more accurate cellular models of human disease.
Martin’s team used almost 2,000 siRNAs to "knock down" every gene in the RGCs that works as a kinase. Kinases are major controllers of the many biochemical pathways involved in health and disease, and blocking them one by one can identify kinases that control a certain action, such as preventing diseased RGCs from dying. Knocking down most of the kinase genes had no effect, but one, dual leucine zipper kinase (DLK), had a remarkable effect. Blocking the function of the DLK gene in cell and mouse models of optic nerve injury dramatically increased the survival of RGCs.
This finding gave the Hopkins-NCATS team critical information: DLK was the target they needed to block to prevent RGC death. So they rushed back to the many compounds they had identified using the assay to find compounds that inhibited DLK. The team searched their screening results and information in the NCATS Pharmaceutical Collection, a database of all approved drugs, to identify a compound that prevented RGC death in their assay and was also known to block DLK. They were excited to find that the compound tozasertib had both needed properties, and they predicted that this compound would have the same effect as DLK knockdown, increasing survival of RGCs.
The final step of the translational process was to test this prediction. As they had hoped, the Hopkins-NCATS team found that tozasertib effectively prevented RGC death in rodent models of not only glaucoma but also traumatic optic neuropathy, a condition in which an injury to the optic nerve results in vision loss.
Although hurdles remain before this approach can be used in the clinic, these studies offer a crucial new target for the treatment of widespread diseases that cause blindness. Tozasertib is not a practical treatment candidate for glaucoma patients because it was developed for short-term use to treat cancer and has effects on other kinases that would lead to side effects with long-term use. The Hopkins scientists now are testing additional compounds in hopes of finding one that can specifically target DLK without the side effects of tozasertib. In a related collaboration, funded by the Foundation Fighting Blindness, the Hopkins-NCATS team is working to identify promising drug leads for other retinal degenerative diseases based on the therapeutic strategy developed in this collaboration.
Zack noted that working with Inglese, Martin and their colleagues enabled his scientists to serve as integral partners in the research process. "What made our collaboration a bit different was that we didn’t just hand off our biological assay for another team to screen," Zack said. "Rather, our researchers were intimately involved in the small molecule and RNAi screens. It allowed us to learn from NCATS researchers and vice versa, with each of us gaining some expertise in the process, and the complementarity of the collaboration led to a more successful outcome."
"This remarkable story illustrates NCATS principles and impact beautifully," said NCATS Director Christopher P. Austin, M.D. "The collaborative research team came together to solve a problem neither could solve alone. They developed multiple new methods to overcome sequential translational roadblocks, demonstrated their effectiveness in glaucoma and disseminated those advances in publications. These innovative approaches now promise to benefit many other researchers seeking to identify molecular targets and potential therapeutic strategies in a wide spectrum of diseases." Winners will be invited to speak at the 2013 DREAM conference held November 8–12 in Toronto, Canada.
Posted August 2013