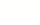Our Impact on Drug Discovery and Development
Our research teams develop and apply cutting-edge approaches that speed therapeutic solutions for unmet health needs.
Moving More Treatments from the Lab to the Clinic
A new drug’s journey from the lab to the medicine cabinet can take up to 15 years. On the way, it passes through dozens of steps — and potential failure points. As a result, many never get approved for human use.
For more than 10 years, we’ve been making the drug discovery and development steps faster and more predictable. We built a pharmaceutical collection of nearly every drug approved for humans. We also built automated solutions to swiftly test drugs or combinations of them for new uses, including emerging infectious diseases. Through our partnerships with academia, industry and patient advocacy groups, we have enabled more than 45 promising new drugs to move into clinical trials.
Our newer drug discovery and development efforts focus on preparing for future pandemics and building a one-stop shop for finding, designing and testing new molecules with therapeutic potential.
Impact Stories
New Screening Tool Could Rapidly Reveal Better Cancer-Drug Combinations
Scientists at NCATS and NCI designed a screening method that could help predict in the lab how combos of three or more cancer drugs will work in people.
NCATS Enables IND Clearances and Drug Approvals
Our labs play a critical, hands-on role in getting new therapies to patients by partnering to discover and develop drugs for rare diseases and other unmet medical needs.
Drug Discovery and Development Research Activities
We speed preclinical research on promising new medicines through a variety of research activities.

Preclinical Chemical Biology Laboratory
Our scientists and collaborators bridge chemistry and the biology underlying disease to develop new ways to discover and create chemical probes and potential drug candidates.
Matrix Combination Screening
Our experts use matrix combination screening technology to quickly identify promising drug combinations with the most potential to help patients.

Compound Management
Our compound management team uses sophisticated and automated techniques to supply chemicals for NCATS screening experiments to uncover new treatments for diseases.
Drug Discovery and Development Research News

NIH Combines Traditional High-Throughput Screening and Artificial Intelligence to Fast-Track Probe Discovery for ALDH Enzymes
January 6, 2026 - NCATS News
- Compound Management
- Our Impact on Drug Discovery and Development
NIH researchers developed a strategy combining artificial intelligence and high-throughput screening to identify chemical candidates for multiple members of aldehyde dehydrogenase (ALDH) protein family, which play roles in metabolism and disease.
Read ArticleTB Alliance and Lupin Announce Collaboration to Advance and Commercialize Telacebec for Multiple Neglected Diseases
January 30, 2026 - Grantee/Partner News
- Our Impact on Drug Discovery and Development
NIH Combines Traditional High-Throughput Screening and Artificial Intelligence to Fast-Track Probe Discovery for ALDH Enzymes
January 6, 2026 - NCATS News
- Compound Management
- Our Impact on Drug Discovery and Development
NIH researchers developed a strategy combining artificial intelligence and high-throughput screening to identify chemical candidates for multiple members of aldehyde dehydrogenase (ALDH) protein family, which play roles in metabolism and disease.
How Academic Researchers Can Use Patent Data to Fuel Drug Repositioning Research
December 17, 2025 - Grantee/Partner News
- Bridging Interventional Development Gaps (BrIDGs)
- Our Impact on Drug Discovery and Development